In our study, two limiting approaches, perfect liquid-phase mixing
and pure diffusion, are adopted to pursue the water-absorption
analysis. The corresponding results are summarized here.
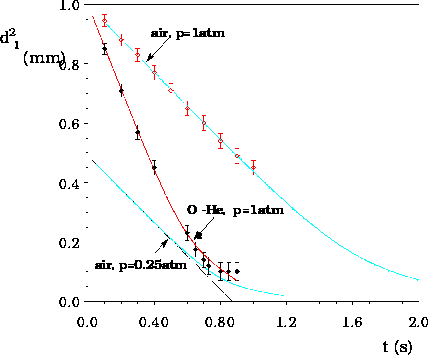
The square of the droplet diameter as a function of time for
droplets initially 1 mm in diameter, burning in air at pressure
p = 1 atm and
temperature T2 = 300 K and in a helium-oxygen mixture with
the oxygen mole fraction
of 0.5 at p = 1atm and T2 = 300 K; and for a droplet initially
0.7 mm in diameter, burning in air at p = 0.25 atm and T2 = 300 K;
solid lines are for perfect liquid-phase mixing, dashed line are for
time-dependent liquid-phase diffusion, points are experimental data
from literature:

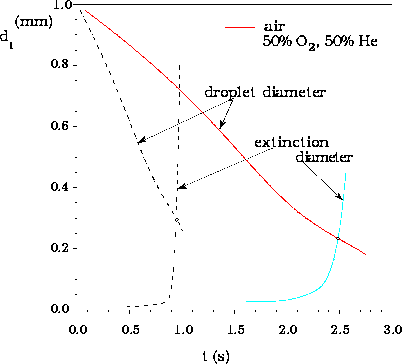
The droplet diameter and the extinction diameter as functions of time with time-dependent water absorption for perfect liquid-phase mixing, for droplet initially 1 mm in diameter, burning in two different atmospheres at p = 1 atm and T2 = 300 K, illustrating how extinction conditions are determined:
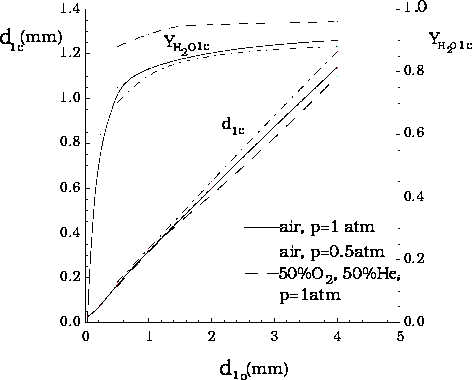
The extinction diameter and the water mass fraction in the liquid at extinction, as functions of initial droplet diameter, for perfect liquid-phase mixing, for burning in three different atmospheres at T2 = 300 K: